If someone you know has ever been diagnosed with cancer, they likely received a treatment known as chemotherapy, or “chemo”, which is the use of drugs to kill cancer cells or stop them from growing [1]. Depending on the person’s individual cancer, chemotherapy could be given alone or in combination with surgery and/or radiation. Some early-stage cancers may not even need chemotherapy at all! But what exactly is chemotherapy, and how does it work?
Chemotherapy drugs are designed to be as specific to cancer cells as possible, in order to minimize harm to the normal cells in our bodies. Cancer cells are usually characterized by abnormal, uncontrollable growth and division, whereas most of our normal cells divide rarely or not at all. Cancer cells do this by learning how to ignore OFF signals that tell normal cells when to stop dividing or to die, and/or by learning how to divide without requiring any ON signals [2]. As a result, most chemotherapy drugs target cellular pathways that control cell growth and division. The four major subtypes of chemotherapy drugs are:
Alkylating agents
Antimetabolites
Anti-cancer antibiotics
Microtubule Inhibitors
(1) Alkylating Agents
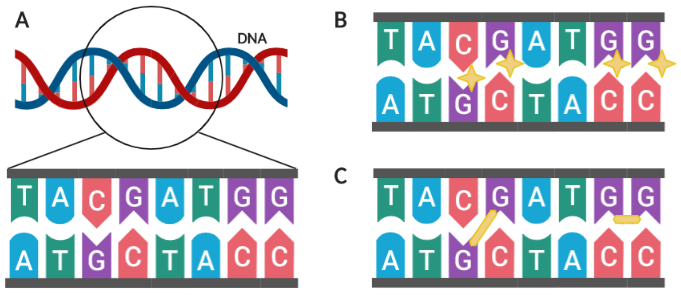
Figure 1. Alkylating agents modify DNA at G nucleotides. (A) Close-up view of nucleotide pairs in a DNA strand. (B) Alkylating agents (depicted as yellow stars) modify G nucleotides. (C) Alkylating agents may form crosslinks (depicted as yellow lines) between or within DNA strands. Created in BioRender.
The instructions for how our cells function and grow are written in our DNA, which is composed of two strands of genetic material connected by molecular bonds. Each strand is composed of a unique sequence of four “letters” (A, T, C G), referred to as nucleotides (Figure 1A). When cells divide, this genetic information is read by specialized protein machinery and duplicated, with each of the two resulting cells receiving one complete copy of your DNA.
Alkylating agents bind to and modify nucleotides—specifically guanine, or the letter “G”—in our DNA and prevent them from being recognized properly during DNA duplication (Figure 1B) [3]. Consequently, mistakes (known as mutations) are introduced and passed on to the newly formed daughter cells. Since cancer cells divide so frequently and do not respond to damage-sensing OFF signals, mutations build up at a much higher rate in cancer cells than normal cells and eventually reach critical levels that cause the cancer cells to die. Alkylating agents are named after the specific molecule (known as an alkyl group) being added onto the nucleotides. If two alkyl groups are present on the same alkylating agent, each group can bind to their own guanine nucleotide and form crosslinks, either between strands (inter-strand) or within the same strand (inter-strand; Figure 1C). Common examples of alkylating agents are cyclophosphamide and iomustine.
Some chemotherapeutic drugs without alkyl groups are also able to form crosslinks and are therefore loosely classified as alkylating agents. The most common examples of non-alkylating crosslinking agents are platinum-containing compounds, such as cisplatin and carboplatin, which are used extensively in the treatment of ovarian cancer.
(2) Antimetabolites
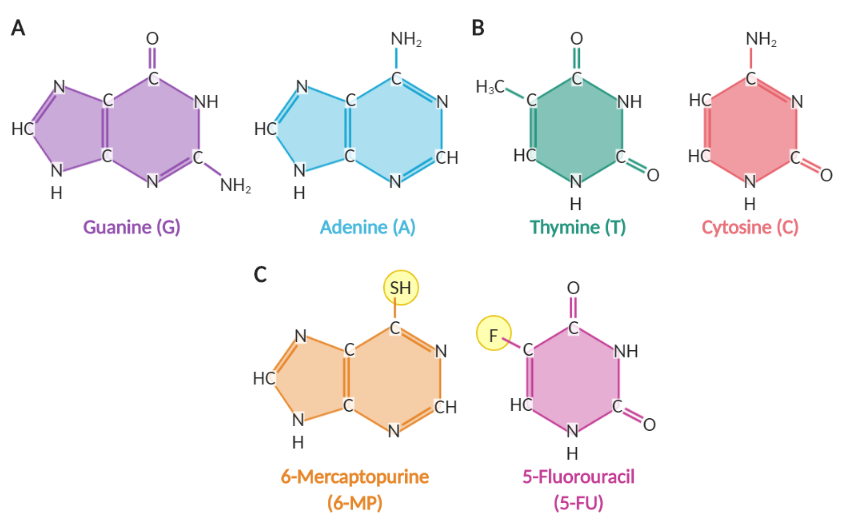
Figure 2. Nucleotide bases and antimetabolites.(A) Purine nucleotide bases. (B) Pyrimidine nucleotide bases. (C) Two antimetabolites: 6-Mercaptopurine and 5-Fluorouracil. Modifications from normal nucleotide bases are circled in yellow. Created in BioRender.
The “letters”, or nucleotides, that make up our DNA sequence can be split into two categories based on their shape: purines (two rings; Figure 2A) and pyrimidines (one ring; Figure 2B). Small chemical modifications on these structures result in production of antimetabolites, which are mistaken by the cell for normal compounds involved in the production of nucleotides [4]. Because antimetabolites are only effective against cells that are preparing to divide, they are much more likely to cause damage to cancer cells, which divide frequently. Antimetabolites can have one of three methods of action:
Inhibit key cellular machinery required for making nucleotides
If antimetabolites mimic the shape of intermediate compounds in the production of normal nucleotides, they can irreversibly bind to functional proteins (enzymes) and prevent them from working properly. An example is methotrexate, which binds to an enzyme involved in producing the T letter in DNA; DNA can no longer duplicate itself if there are no T nucleotides available to use.
Become erroneously incorporated into DNA and cause mutations
When antimetabolites mimic the shape of the A, T, C, or G nucleotides, they can be accidentally added to DNA strands when they are being duplicated. When these cells divide again, the cellular machinery will not be able to properly recognize the antimetabolites and will add an incorrect nucleotide in that position, creating a mutation. Examples of drugs in this category are 6-Mercaptopurine (6-MP) and 5-Fluorouracil (5-FU), which are modified by enzymes in the body and incorporated into DNA (Figure 2C).
Cause premature termination of DNA replication and DNA breaks
Normal nucleotides have a specific molecular structure that allows them to be joined together during replication. When antimetabolites are missing this structure, DNA replication stops as soon as the antimetabolite is incorporated into DNA and is unable to resume. Example: cytarabine (AraC).
(3) Anti-Cancer Antibiotics
If you have ever had a bacterial infection, you likely would have been prescribed antibiotics by your doctor. It is important to note that these antibiotics are only effective against bacteria and not cancer; likewise, anti-cancer antibiotics are not effective against bacteria.
Anti-cancer antibiotics damage DNA. Because cancer cells tend to ignore OFF signals that tell the cell to stop dividing when damaged, they quickly accumulate a high volume of mutations and DNA breaks, eventually resulting in cell death. One example of an anti-cancer antibiotic is doxorubicin, which is converted in the body into an unstable, toxic molecule that releases reactive oxygen species (ROS) [5]. ROS can react with pretty much any molecule in the cell (including DNA and important functional proteins) and damage them, disrupting normal cellular processes and creating breaks in the DNA strands. The accumulation of high levels of damage eventually triggers programmed cell death.
Anti-cancer antibiotics are less selective for cancer cells than other types of chemotherapy and are more likely to affect normal cells. Thus, it is important to monitor any adverse side effects experienced by the patients and to stop the treatment when the damage to normal tissues becomes too high.
(4) Microtubule Inhibitors
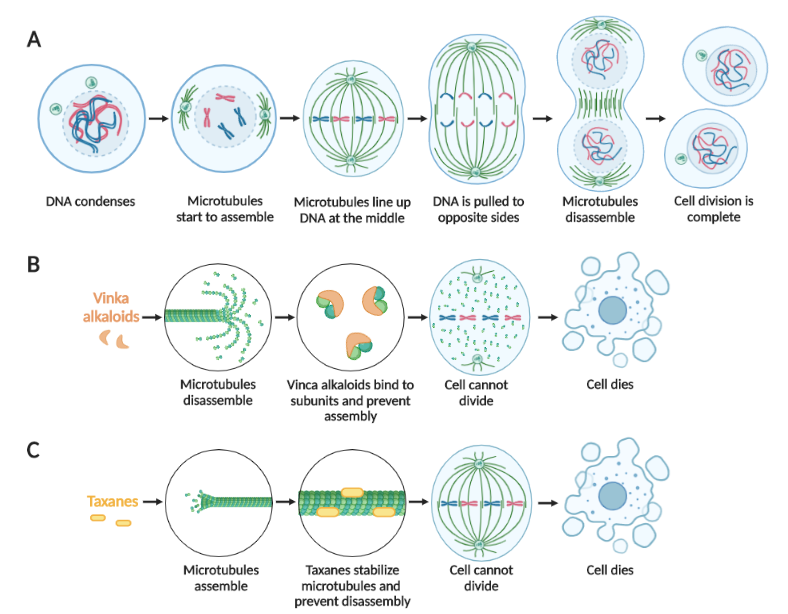
Figure 3. Cell division is prevented by microtubule inhibitors.(A) The sequence of normal cell division. Treatment with vinca alkaloids (B) or taxanes (C) prevents cell division and results in cell death. Created in BioRender.
When cells divide in two, there are specialized molecular strands known as microtubules which help sort the duplicated DNA into portions for each daughter cell (Figure 3A). When these strands are inhibited, there are two common outcomes:
DNA is distributed incorrectly, resulting in large-scale DNA abnormalities
Cell division is blocked altogether, resulting in death of both daughter cells
Because microtubule inhibitors take effect when the cell is in the middle of dividing, they are more likely to affect cancer cells, rather than normal cells.
There are two main types of microtubule inhibitors [6]. The first category consists of vinca alkaloids, such as vincristine, which cause the microtubule strands to break down into individual subunits and prevent them from re-forming (Figure 3B). As a result, no machinery exists to distribute the divided DNA and both copies remain in a single cell; too many copies of the DNA results in programmed cell death. The second category of microtubule inhibitors is taxanes, such as paclitaxel, which freeze the microtubule strands in place and prevent them from breaking down (Figure 3C). Consequently, the cell cannot divide properly and undergoes cell death.
Why does chemotherapy make us so sick?
Although cancer cells are much more likely to be dividing than our normal cells, there are specific cell types in our body that must divide on a regular basis. The most notable example is your hair follicle cells, which divide in order to make your hair grow. As a result, it is common for patients receiving chemotherapy to lose their hair. The cells that make up the lining of your stomach and intestines also divide frequently; as a result, patients receiving chemotherapy often are nauseous or sick to their stomach. Because the chemotherapy drugs cannot differentiate between cancer cells and normal dividing cells, these normal cells also die as an unwanted consequence. Doctors prescribe specific chemotherapy drugs that are best suited for each patient’s individual cancer type, while monitoring the patient for adverse side effects and adjusting the dose or type of medication accordingly.
The drugs presented in this article are just a small subset of chemotherapy drugs available. Recent advances in medical research have also led to the development of more specific, personalized cancer therapies which target much more specific characteristics of cancer cells. As we continue to learn more about cancer and its development, scientists and doctors can continue to minimize adverse side effects and achieve the best possible outcome for cancer patients.