Last month, I wrote a post called How your cells stop cancer before it starts, which focused on the fact that, despite us being exposed to DNA-damaging events pretty much all the time, we are not all walking bags of cancer. I then touched on a few reasons why this is the case, including some self-correcting and protective cellular mechanisms that keep track of our DNA; the fact that you need several mutations, in just the right places, to start a cancer growing; and that when cells do start to accumulate potentially dangerous DNA damage events, they are sometimes seen and eliminated by your immune system, which can kill a potential tumour before it gains a foothold.
But how exactly does your immune system recognize a damaged cell? After all, damaged or not, those cells are still you. Your immune system is specifically evolved to recognize and kill things that are not-you, like bacteria and viruses, and leave the you part of your body alone. Immune surveillance of damaged cells can be important for stopping cancer before it starts. But if your immune system isn’t supposed to recognize your own cells in the first place, how do DNA-damaged cells signal to the immune system at all? There are several answers to this, and this month I was lead author on an article published in Essays in Biochemistry, where we reviewed a few ways in which the immune system can be alerted to DNA damage. If you don’t feel like reading a 3500-word essay that was written for an audience of career cell biologists, consider this your SparkNotes. I’ll even give the ending away: It’s all about the micronucleus.
A micronucleus is exactly what it sounds like: a tiny nucleus. The nucleus of a cell is where your entire genome is stored: all of the DNA that provides the necessary instructions for your cells to do their jobs. When cells go through mitosis, they copy their DNA and break down the nuclear envelope that encloses the genome. Then, cellular machinery called the mitotic spindle comes in like a grappling hook, grabbing each of the chromosomes by their centromere, which is a structure near the chromosome’s middle. The mitotic spindle pulls the two copies of the genome away from each other by their centromeres, to opposite sides of the dividing cell. The cell then re-forms the nuclei that will end up inside two completely separate, daughter cells, using these pulled-apart chromosome masses as the starting point. But if, at the end of this process, you see not only two full nuclei but also micronuclei, this is like an error message in the cell saying something has gone wrong.
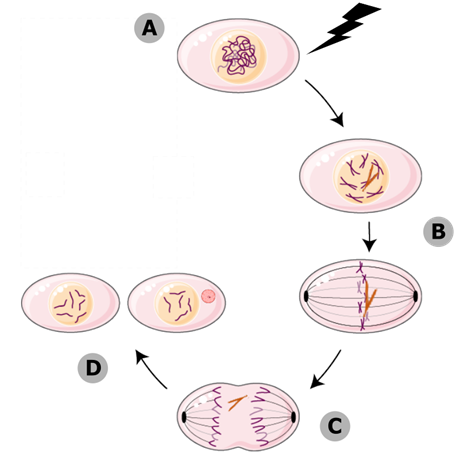
Figure 1: Micronuclei form during mitosis. (A) When cells experience DNA damage, their chromosomes can become broken. (B) If the broken chromosomes (orange) aren’t repaired, they stay in the nucleus until mitosis. (C) One half of the break doesn’t have a centromere, so the mitotic spindle can’t attach to it, and it gets left behind as the rest of the genome is pulled to the cell poles. (D) The broken fragment gets sequestered into its own micronucleus, completely separate from the daughter nuclei.
Micronuclei form when a cell doesn’t repair double-stranded breaks before going through cell division. If a double-stranded break hasn’t been resolved, that means one of your chromosomes is in two pieces. Only one of those pieces contains the centromere, which is the part the mitotic spindle grabs on to. When the two copies of the genome get pulled apart to the cell poles, where the daughter nuclei are going to form, a tiny piece of centromere-less DNA is going to get left behind. While the rest of the genome has a new nuclear envelope forming around it, that broken chromosomal fragment recruits its own tiny envelope, entirely separate, and sequesters itself into a micronucleus. You can see these micronuclei when you look at cells under a microscope. They tell you that the cell was dealing with a lot of DNA damage, and didn’t fix it all before it started to make copies of itself.
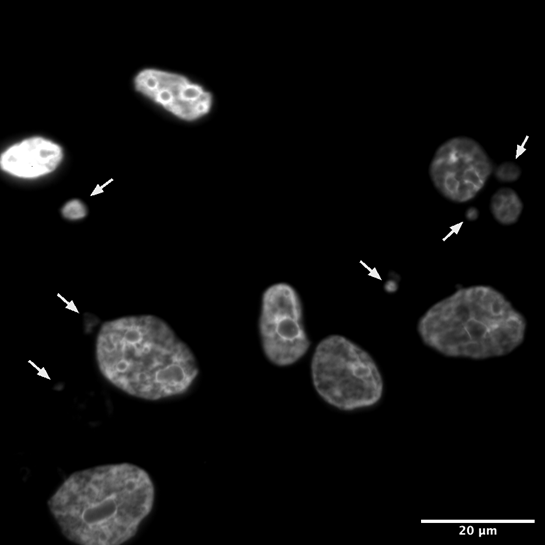
Figure 2: Human cells under a fluorescent microscope, treated with a dye that binds DNA. The large objects are nuclei, and the smaller ones pointed out with white arrows are micronuclei.
Micronuclei are one of the tools at your cell’s disposal for broadcasting DNA damage and getting the immune system to intervene. They do this in two major ways: First, they can pretend to be viruses, and get the immune system to treat the DNA-damaged cell like one that’s been infected. Micronuclei are tiny pieces of DNA that live in the cytosol, the part of your cell that’s outside the nucleus, where DNA is never supposed to be. If the hastily assembled micronuclear envelope breaks, the DNA contained within it will be exposed to cytoplasmic proteins. This includes some proteins that are there for the express purpose of detecting viral DNA. These proteins, called viral pattern recognition receptors (PRRs) bind to the micronuclear DNA, and set off the same signaling cascade that would be downstream of infection with a DNA virus. PRRs don’t know that this is your DNA – they just see DNA is the cytosol, scream virus!, and get the immune system to come running.
The second major way that micronuclei can alert an immune response is by exacerbating the level of DNA damage the cell is harbouring, making it impossible for the immune system to miss. Micronuclei are sequestered away from the main nucleus, and that usually means they don’t have access to all the proteins that normally scan your DNA, make sure it’s okay, and fix any damage that they do find. This means that as long as that fragment of DNA hangs out in the micronucleus, it’s vulnerable, and can accumulate a lot of DNA damage. If there are any protein-coding genes on that fragment of micronuclear DNA, then DNA damage is going to severely mutate it. In later rounds of mitosis, when the nuclear envelope breaks down again, micronuclei can sometimes make their way back inside the primary nucleus. Those mutated genes are then going to produce mutated proteins, and proteins get displayed on the outside of all cells, on a molecule called MHC. There are certain types of immune cells that are always patrolling, and they check the proteins displayed on MHC to make sure they’re normal. A cell that’s got a lot of micronuclei is going to be displaying decidedly messed up proteins. The patrolling immune cells see that, become activated, and kill the damaged cell.
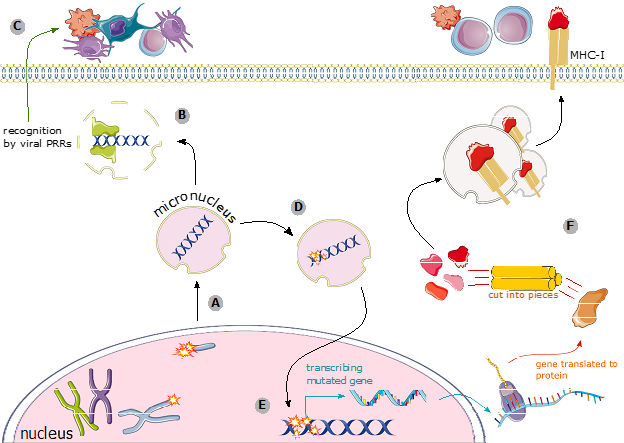
Figure 3: Ways for micronuclei to alert your immune system. (A) When double-stranded breaks aren’t repaired, the piece of the chromosome without a centromere can end up in a micronucleus. (B) If the envelope around the micronucleus breaks, the DNA inside can be recognized by viral PRRs. (D) PRRs initiate viral signaling cascades that alerts the immune system. (D) The DNA inside the micronucleus is vulnerable to irreparable DNA damage, and can accumulate mutations. (E) If that damaged DNA is re-incorporated into the primary nucleus, it could mean that mutated genes are transcribed and translated into mutated proteins. (F) Mutated proteins are loaded onto MHC, and the immune system see them.
Micronuclei form when a cell fails to resolve all of its DNA damage before going through cell division. That’s a process which, if left unchecked, renders the cell extremely vulnerable to acquiring the kinds of mutations that can turn it into a cancer. Micronuclei are special in that they’re a visual readout, telling scientists that the cell is damaged; but they’re also functional, and they can broadcast that damage to the immune system, stopping cancer before it starts. One year ago, I had never even heard of micronuclei. Now, they’re the focus of my PhD. They play such an important role in keeping DNA damage in check, and I can’t wait to learn more about them.